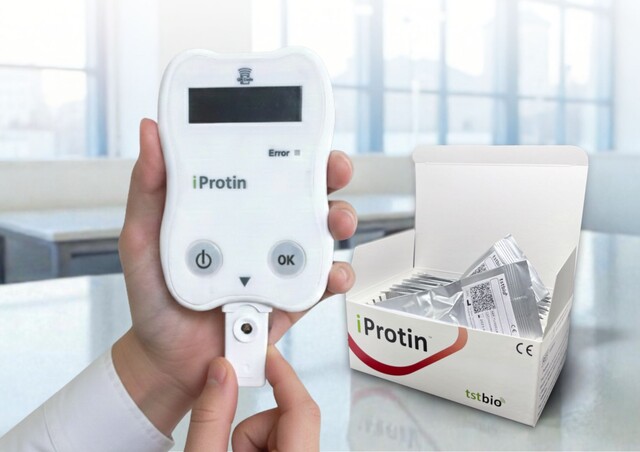
The iProtin® Reader enables precise quantitative measurement of lipoprotein(a), a key biomarker associated with atherosclerotic cardiovascular disease (ASCVD), using Lp(a) assay cassettes based on microchip technology. Using the iProtin® SAW diagnostic platform, the reader employs high-frequency Shear Horizontal Surface Acoustic Wave (SH-SAW) sensor technology to detect antigen-antibody binding in real time – without the need for enzymes or fluorescent labels.
The test requires only 5 µL of blood (capillary blood from a fingertip or heparin-anticoagulated venous blood) to obtain a quantitative Lp(a) value within 40 seconds.
The compact and robust reader is suitable for use in hospitals, outpatient settings, research and clinical studies, as well as routine diagnostics.
Key Features
- Compact and robust system
- Portable & user-friendly
- Requires only 1 single drop of blood
- No additional blood centrifugation or filter procedure required
- Within 40 seconds to result
- CE-marked / IVD-certified
Compatible Test System
- iProtin® Lp(a) Test Kit
- Also available: iProtin® ApoB Test Kit – for the quantitative measurement of apolipoprotein B, a key biomarker for assessing cardiovascular disease (CVD) risk and supporting the evaluation of coronary heart disease (CHD) risk.